Car batteries are the silent workhorses of automotive technology. They not only start the engine but also power lights, electronics, and increasingly, advanced driver-assistance systems. Over two centuries, battery technology has evolved from early experiments with electricity to today’s high-performance lithium–ion packs powering electric vehicles (EVs).
Understanding the development of car batteries provides valuable insight into why certain chemistries dominate, how innovations have shaped performance, and what the future may hold. This article combines the history, functionality, and technological advances of both lead–acid and modern automotive batteries.
- From Ancient Curiosities to the First Confirmed Battery
- Early 19th-Century Foundations
- The Birth of the Lead–Acid Battery
- Faure’s Breakthrough and Industrial Adoption
- From Railways to Automobiles
- Types of Lead–Acid Batteries
- Beyond Starting: Specialised Applications
- The Science Behind Lead–Acid Operation
- Advances in Lead–Acid Technology
- The Rise of Lithium Batteries
- Common Automotive Battery Types Today
- Choosing the Right Battery
- FAQ
- Conclusion
- References:
From Ancient Curiosities to the First Confirmed Battery
One of the most debated archaeological finds is the so-called “Baghdad Battery”, a small ceramic jar discovered in 1936 near Baghdad and dated to roughly 250 BCE – 250 CE. Inside was a copper cylinder encasing an iron rod, with evidence it may have once contained an acidic liquid. In the 20th century, experiments showed this could produce about 1–2 volts.
However, modern scholarly consensus rejects the idea it was truly a battery. No wires, electroplated artefacts, or historical records exist to support this claim. Experts such as Paul Craddock (British Museum) believe it was more likely a storage vessel for scrolls or ritual items.
The first confirmed battery was the Voltaic Pile, invented in 1799 by Alessandro Volta. It used alternating zinc and copper discs separated by brine-soaked cloth to generate a continuous current. This was the true starting point for modern battery science.
Early 19th-Century Foundations
In 1801, French scientist Nicolas Gautherot observed that wires used for electrolysis could produce a “secondary” current after the main supply was removed. Around the same time, German scientist Johann Wilhelm Ritter explored electrode polarisation. These discoveries hinted at the possibility of rechargeable electricity storage, laying the groundwork for future inventions.
In 1802, Scottish chemist William Cruickshank created the first mass-producible battery design, though it was non-rechargeable.
The Birth of the Lead–Acid Battery
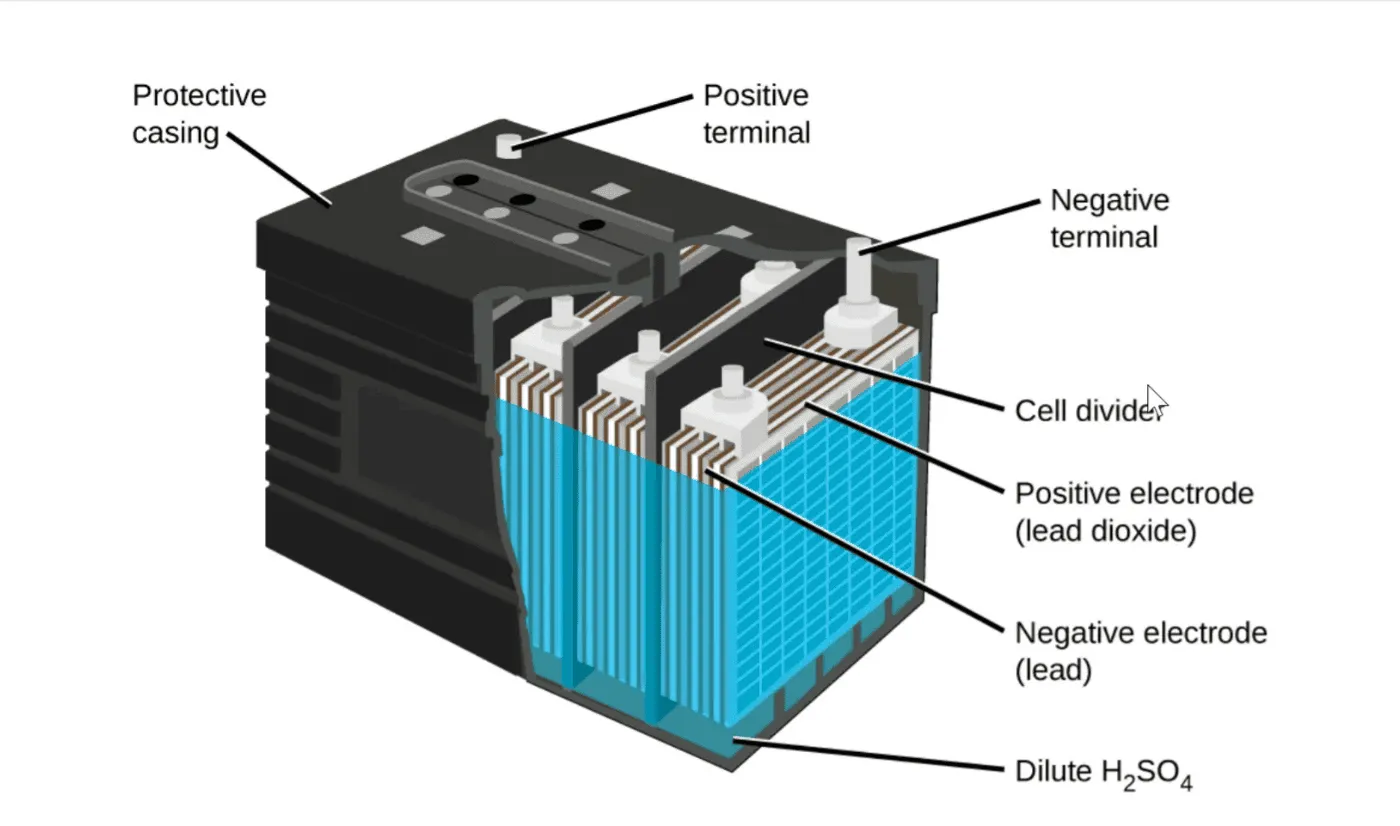
In 1859, Gaston Planté developed the first rechargeable battery — the lead–acid cell. His design used two sheets of lead separated by rubber strips, rolled into a spiral, and submerged in sulphuric acid. When charged, one plate formed lead dioxide (PbO₂), while the other remained as sponge lead (Pb).
Lead–acid chemistry’s unique strength is its ability to deliver high surge currents, making it ideal for starting internal combustion engines.
Faure’s Breakthrough and Industrial Adoption
In 1881, Camille Alphonse Faure introduced a pasted plate construction: a lead grid filled with lead oxide paste. This increased capacity, improved durability, and made large-scale production possible.
By 1886, Henri Tudor was manufacturing lead–acid batteries commercially, leading to their use in vehicles, lighting systems, and industrial applications.

From Railways to Automobiles
Initially used in stationary power systems, lead–acid batteries began powering railway carriage lighting in the 1870s and 1880s. Their automotive breakthrough came in 1912 when Cadillac introduced the first production car with an electric starter. This replaced the dangerous hand crank with a push-button start, driving widespread adoption of lead–acid batteries in cars.
Types of Lead–Acid Batteries
- Starter (SLI) Batteries – Deliver short bursts of high current for engine starting; not suited to deep discharge.
- Micro-Cycle Batteries – Designed for start–stop vehicles, tolerating frequent shallow cycles.
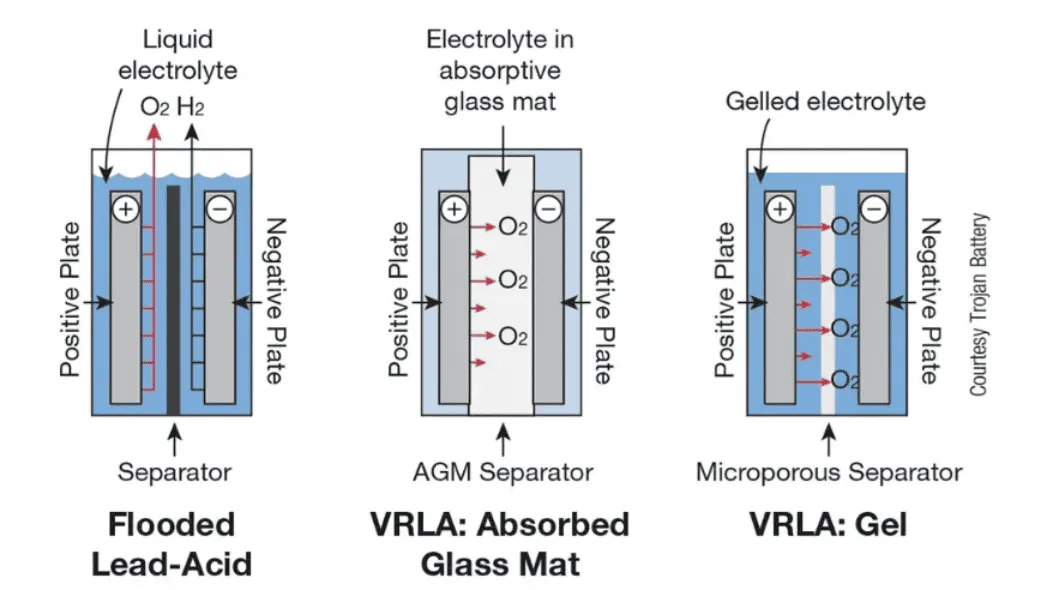
- Flooded Lead–Acid – Traditional, with liquid electrolyte; requires periodic maintenance.
- Absorbent Glass Mat (AGM) – Valve-regulated, spill-proof, and vibration-resistant.
- Gel Batteries – Gelled electrolyte for leak resistance and deep-cycle applications.
Beyond Starting: Specialised Applications
Lead–acid batteries are also used for:
- Deep-Cycle Applications – Marine craft, golf carts, and renewable energy systems.
- Stationary Backup – Data centres, telecom towers, and hospitals.
- Industrial Use – Forklifts and floor-cleaning machines.
The Science Behind Lead–Acid Operation
Discharge:
- Pb (negative plate) + H₂SO₄ → PbSO₄ + 2e⁻
- PbO₂ (positive plate) + H₂SO₄ + 2e⁻ → PbSO₄ + H₂O
Charge:
- The reactions reverse under an external charging current, restoring Pb and PbO₂.
Sulphuric acid acts as the electrolyte, directly contacting both plates. A fully charged cell has a voltage of around 2.04 V under standard conditions, rising slightly depending on acid concentration (as explained by the Nernst equation).
Advances in Lead–Acid Technology
- VRLA (Valve-Regulated Lead–Acid) batteries developed in the 1970s include AGM and gel types, reducing maintenance and improving safety.
- Carbon-enhanced plates improve deep-cycle life and charging speed.
- Bipolar designs aim to reduce weight and increase energy efficiency.
- Improved alloys resist corrosion and reduce water loss.
The Rise of Lithium Batteries
By the 1990s, demand grew for lighter, longer-lasting, higher-capacity batteries — particularly for portable electronics and electric vehicles. Lithium–ion technology delivered:
- Higher energy density.
- Longer cycle life.
- Faster charging.
- Lower weight.
Lithium–ion quickly became the dominant chemistry in EVs and hybrids. Variants such as Lithium Iron Phosphate (LiFePO₄) are prized for safety and thermal stability, particularly in buses and some passenger EVs.
Common Automotive Battery Types Today
- Lead–Acid – Reliable and affordable; ideal for starting.
- AGM Lead–Acid – Maintenance-free with higher performance.
- Gel Lead–Acid – Best for deep-cycle, vibration-prone environments.
- Lithium–Ion – High energy density for EVs.
- NiMH – Used in some hybrids; robust but less energy-dense than lithium–ion.
Choosing the Right Battery
- Match Capacity to Needs – Follow manufacturer amp-hour and CCA specs.
- Check Fit – Ensure correct physical dimensions.
- Consider Climate – AGM and LiFePO₄ handle extremes better; heat shortens flooded lead–acid life.
- Safety – Look for leak-proof and vibration-resistant designs.
- Budget vs Longevity – Lithium–ion costs more but lasts longer; lead–acid is cheaper but shorter-lived.
FAQ
Conclusion
From the disputed Baghdad Battery to Volta’s pioneering Voltaic Pile, from Planté’s spiral-wound lead plates to modern lithium–ion packs, battery technology has transformed mobility and energy storage.
The lead–acid battery’s chemistry remains fundamentally unchanged for over 165 years, yet refinements in materials and design keep it competitive. Lithium–ion has redefined electric mobility, but lead–acid continues to dominate in starting, backup, and cost-sensitive applications.
As automotive technology advances, both chemistries — and new hybrids — will play vital roles in powering the next generation of transport.
References:
1. Eggert, G. (1996). The Enigmatic “Battery of Baghdad”. Skeptical Inquirer, May–June 1996, 31–33.
2. Pavlov, D. (2017). Lead–Acid Batteries: Science and Technology. eBook ISBN: 9780444595607
3. Moseley, P. T., Garche, J., Parker, C. D., & Rand, D. A. J. (Eds.). (2004). Valve-regulated lead–acid batteries (1st ed.).
4. Yoshino, A. (2012). The birth of the lithium-ion battery. Angewandte Chemie International Edition, 51(24), 5798–5800. https://doi.org/10.1002/anie.201105006

