Since their invention in 1859 by Gaston Planté, lead–acid batteries have remained among the most reliable and cost-effective electrochemical storage technologies. At the heart of their performance lies the battery plate — the key component responsible for storing and releasing electrical energy through reversible electrochemical reactions.
While consumers often focus on capacity or cold cranking amps (CCA), manufacturers and engineers know that the composition, structure, and processing of plates largely determine a battery’s longevity, energy density, and cycle life.
This article examines the science, manufacturing methods, and modern innovations shaping lead–acid plate production for automotive, industrial, and renewable energy markets.
1. Key Components of Lead–Acid Battery Plates
Lead–acid battery plates are designed to optimise electrochemical reactivity, mechanical strength, and corrosion resistance. Each plate consists of:
- Positive Plate – Lead dioxide (PbO₂), a strong oxidising agent accepting electrons during discharge.
- Negative Plate – Sponge lead (Pb), donating electrons in the discharge process.
- Grid – A lead–antimony, lead–calcium, or hybrid alloy framework acting as both structural support and current collector.
- Active Material Paste – A mixture of leady oxide, water, sulphuric acid, and additives, engineered to maximise porosity and surface area for rapid ion transport.
Plate Variants:
- Flat Plates – Predominantly used in automotive starting, lighting, and ignition (SLI) batteries.
- Tubular Plates – Preferred for deep-cycle applications due to superior active material retention.
2. Manufacturing Process: From Lead to Active Plate
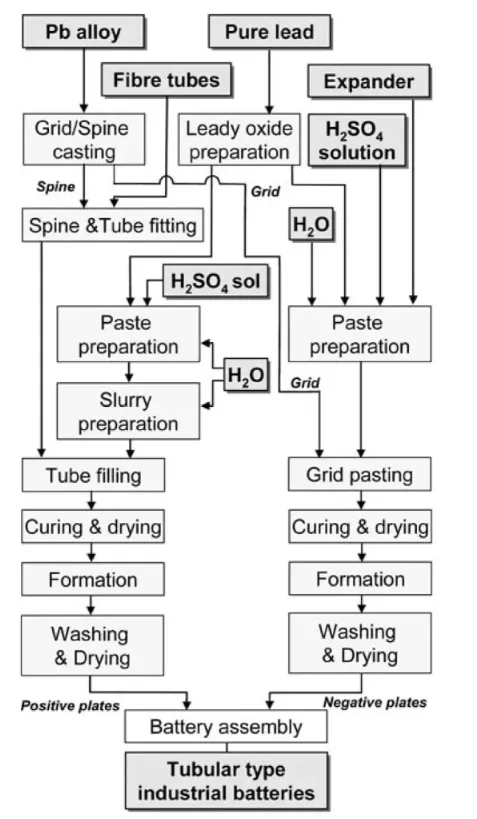
The production of lead–acid battery plates requires precision engineering to balance capacity, cycle life, and efficiency.
2.1 Leady Oxide Production
Two main industrial processes are used:
- Ball Mill Method – Solid lead ingots are ground in a rotating drum with forced air circulation, producing particles 5–50 µm in size for an optimal balance between initial capacity and longevity.
- Barton Pot Method – Molten lead is atomised in an oxidising atmosphere, allowing finer control of particle morphology and oxidation states, enhancing paste adhesion.
Both methods now incorporate dust extraction and closed-loop recycling systems to minimise lead emissions, meeting ISO 14001 environmental compliance and OSHA lead exposure limits.
2.2 Paste Mixing and Additive Optimisation
Leady oxide is combined with water, sulphuric acid, and functional additives such as:
- Carbon Black – Improves charge acceptance and partial-state-of-charge (PSoC) performance in stop–start vehicles.
- Fibrous Reinforcements – Reduce active material shedding in high-vibration environments.
- Barium Sulphate – Acts as a seed crystal to improve PbSO₄ distribution during formation.
Precise rheological control of the paste ensures uniform coating and optimal electrolyte penetration.
2.3 Grid Casting and Pasting
- Grids are cast from lead–calcium alloys for maintenance-free batteries or lead–antimony alloys for deep-cycle durability.
- Automated pasting machines apply paste under controlled pressure to maximise adhesion.
2.4 Curing and Formation
- Curing in humidity-controlled chambers converts part of the paste into basic lead sulphates, improving structural integrity.
- Formation uses controlled electrochemical charging to transform precursors into PbO₂ (positive plates) and sponge lead (negative plates), establishing porosity profiles for efficient electrolyte access.
3. Factors Influencing Plate Performance
Plate performance depends on electrochemical kinetics and mechanical stability:
- Particle Size Distribution – Medium particle sizes (~10–30 µm) extend cycle life; smaller sizes boost initial capacity but degrade faster.
- Porosity and Surface Area – Higher porosity enables higher discharge rates but must be balanced with mechanical strength.
- Phase Composition – High α-PbO₂ content enhances corrosion resistance in positive plates.
- Grid Alloy Choice – Calcium alloys reduce water loss but have lower overcharge tolerance; antimony alloys support deep cycling but require periodic water topping-up.

4. Applications by Plate Type
Flat Plates
- Automotive SLI batteries – Designed for short, high-current bursts.
- UPS systems – Provide instant backup for critical electronics.
Tubular Plates
- Renewable energy storage – Superior cycling for solar/wind applications.
- Industrial equipment – Forklifts, electric pallet trucks, and floor scrubbers.
5. Modern Innovations in Plate Technology
- Carbon-Enhanced Negative Plates – Improve dynamic charge acceptance in micro-hybrid vehicles.
- Bipolar Plate Configurations – Reduce weight and increase energy density for hybrid EVs.
- AI-Optimised Curing Profiles – Machine learning models adjust curing parameters for alloy/paste combinations.
- Recycled Lead Integration – Over 85 per cent of lead in modern batteries now comes from closed-loop recycling.
Frequently Asked Questions (FAQs)
Conclusion
Lead–acid battery plates remain central to performance, lifespan, and application suitability. Advances in paste chemistry, grid alloys, and formation technology are extending the viability of this century-old invention into new markets, including micro-hybrids and renewable storage.
Through material science and sustainable manufacturing, the industry continues to balance performance and environmental responsibility, ensuring lead–acid batteries maintain their global relevance.
References:
1. Pavlov, D. (2017). Lead–Acid Batteries: Science and Technology. eBook ISBN: 9780444595607
2. International Electrotechnical Commission. (2018). IEC 60095-1: Lead-Acid Starter Batteries – Part 1: General Requirements and Methods of Test. IEC. Part 1: General Requirements and Methods of Test. IEC.

