Why Long-Term Storage Damages Lead–Acid Batteries – Causes, Effects, and Best Practices
Lead–acid batteries remain a reliable choice for vehicles, industrial equipment, and backup power systems. However, prolonged storage without proper maintenance can cause irreversible damage — reducing both performance and service life.
Whether stored for seasonal vehicle use, as a spare, or during shipment, lead–acid batteries undergo electrochemical changes even when not in use. This guide explains why long-term open-circuit storage is harmful, the key degradation mechanisms, and practical steps to minimize damage. The recommendations follow international guidance from IEC 60095-1 and EN 50342-6 for automotive and stationary lead–acid batteries.
1. Why Storage Harms Batteries
When a lead–acid battery is stored in an unused, open-circuit state, it begins to lose charge through self-discharge. This natural process slowly reduces the available capacity, eventually making the battery unable to start a vehicle or power equipment.
For example, a car stored in a garage for several months may fail to start, even if the battery was new before storage. Prolonged neglect accelerates capacity loss, shortens service life, and can lead to complete failure.
2. Key Factors in Battery Damage
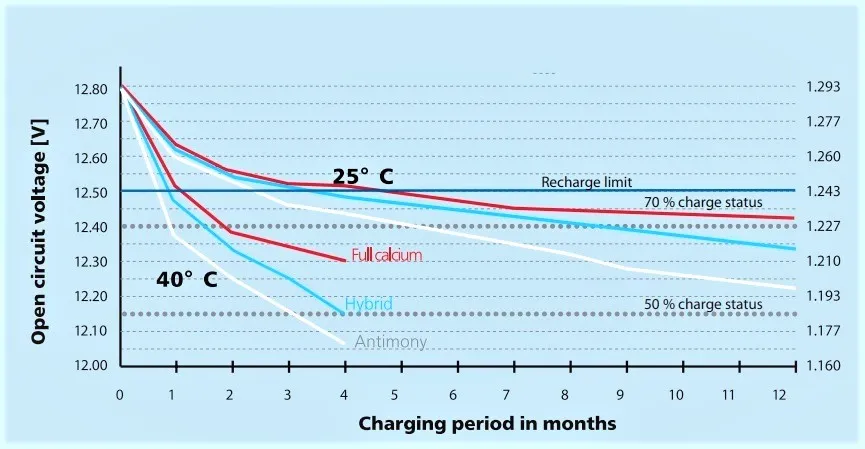
Self-Discharge
- At 20–25 °C: Lead acid batteries lose 3–7% of their charge per month.
- At high temperatures (up to 50 °C): Self-discharge rates can double to 10–15% per month, due to faster internal chemical reactions (Arrhenius effect).
Sulphate ion
- When stored at low charge, lead sulfate (PbSO₄) crystals harden on the plates.
- This reduces active surface area, lowering capacity and cold cranking amps (CCA) .
- Severe sulphation is largely irreversible, even after recharging.
Electrolyte Evaporation
- In flooded (wet cell) batteries, high temperatures accelerate water loss through gassing.
- Reduced electrolyte levels expose the plates, causing permanent damage and higher internal resistance.
Corrosion
- Terminal oxidation and internal grid corrosion continue during storage, especially in humid or hot environments.
- Older batteries (>3 years) typically lose charge faster due to increased internal leakage currents.
3. Best Practices for Battery Storage
Store in Cool Conditions
- Ideal temperature: 0-15°C to slow self-discharge (3% per month vs. 10% at 40°C).
- Avoid freezing: A fully charged lead–acid battery can withstand down to –60 °C, but a fully discharged one can freeze near –7 °C.
Maintain Charge
- Recharge every 3–6 months to prevent deep discharge (<10.50 V for a 12 V battery).
- Use a smart charger following manufacturer guidelines (typically 14.4 V for 12 V flooded batteries).
Disconnect from Vehicle
- Remove the negative cable to eliminate parasitic drain from electronics such as alarms and GPS trackers.
Use Sealed Battery Designs
- AGM (Absorbent Glass Mat) or gel batteries resist electrolyte loss and require less maintenance in high-heat environments.
Keep Terminals Clean
- Clean and protect terminals with dielectric grease to reduce corrosion and ensure good electrical contact.
Store at 80-100% State of Charge
- Fully charged voltage for a 12V battery: 12.6-12.8V.
- Storing partially charged batteries accelerates sulphation.
4. Conclusion
Long-term storage without proper maintenance can damage lead–acid batteries through self-discharge, sulphation, and electrolyte evaporation — especially in hot climates.
By storing batteries in cool conditions, maintaining their charge, and using sealed designs where appropriate, you can extend service life (typically 3–5 years for automotive batteries) and ensure reliable performance when needed.
Following these best practices, supported by international standards, will keep your vehicle or equipment ready to start — whether after a season, a year, or longer.
FAQs (Frequently Asked Question)
References
- IEC 60095-1:2021 – Lead-acid starter batteries – Part 1: General requirements and methods of test.
- EN 50342-1:2015+A1:2018 – Lead-acid starter batteries – Part 1: General requirements and methods of test
- Pavlov, D. (2011). Lead–Acid Batteries: Science and Technology. Elsevier. ISBN: 9780444528827.
- BCI Battery Service Manual – Technical Application Guide. Battery Council International.
- SAE J537:2023-09 — Storage Batteries. SAE International.

