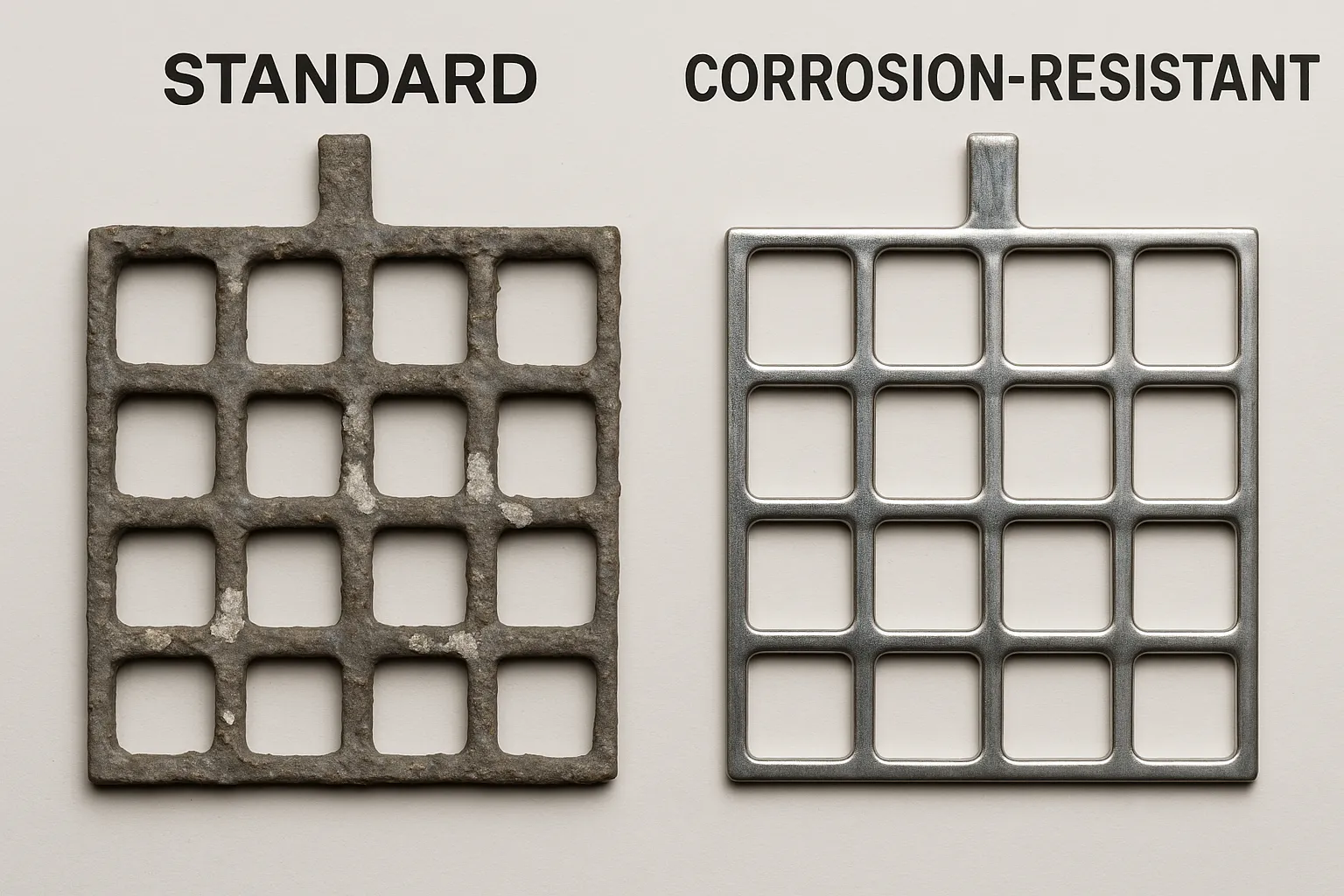
The Hidden Threat of Grid Corrosion
At the core of every lead-acid battery lies its grid—a vital framework that powers vehicles, industrial equipment, and renewable energy systems. But corrosion, like rust eating away at metal, silently weakens these grids, leading to sluggish starts, frequent maintenance, and early failures.
Advanced grid alloys, blending high-purity lead with tin, calcium, and selenium, create batteries that last longer, perform reliably, and save costs for drivers, fleet managers, and industries.
What Is a Battery Grid?
A battery grid is like the skeleton of a lead-acid battery, supporting the active materials (lead dioxide and sponge lead) and conducting electricity. It ensures efficient power delivery and structural stability.
Key Roles
• Conducts Electricity: Carries current during charging and discharging.
• Supports Materials: Holds active materials in place.
• Enables Reactions: Facilitates chemical processes for energy storage.
A weak grid means a weak battery, unable to deliver consistent power.
Why Corrosion Hurts
Corrosion erodes grids, causing cracks and reduced conductivity. For drivers, this means unreliable starts. For businesses, it leads to downtime and higher costs.
Impacts of Corrosion
• Shorter Battery Life: Grids fail faster, cutting lifespan.
• Poor Performance: Less power for starting or heavy loads.
• Higher Costs: More maintenance and replacements add up.
Imagine a corroded grid as a rusty chain—brittle and prone to breaking—while advanced alloyed grids stay strong and smooth.
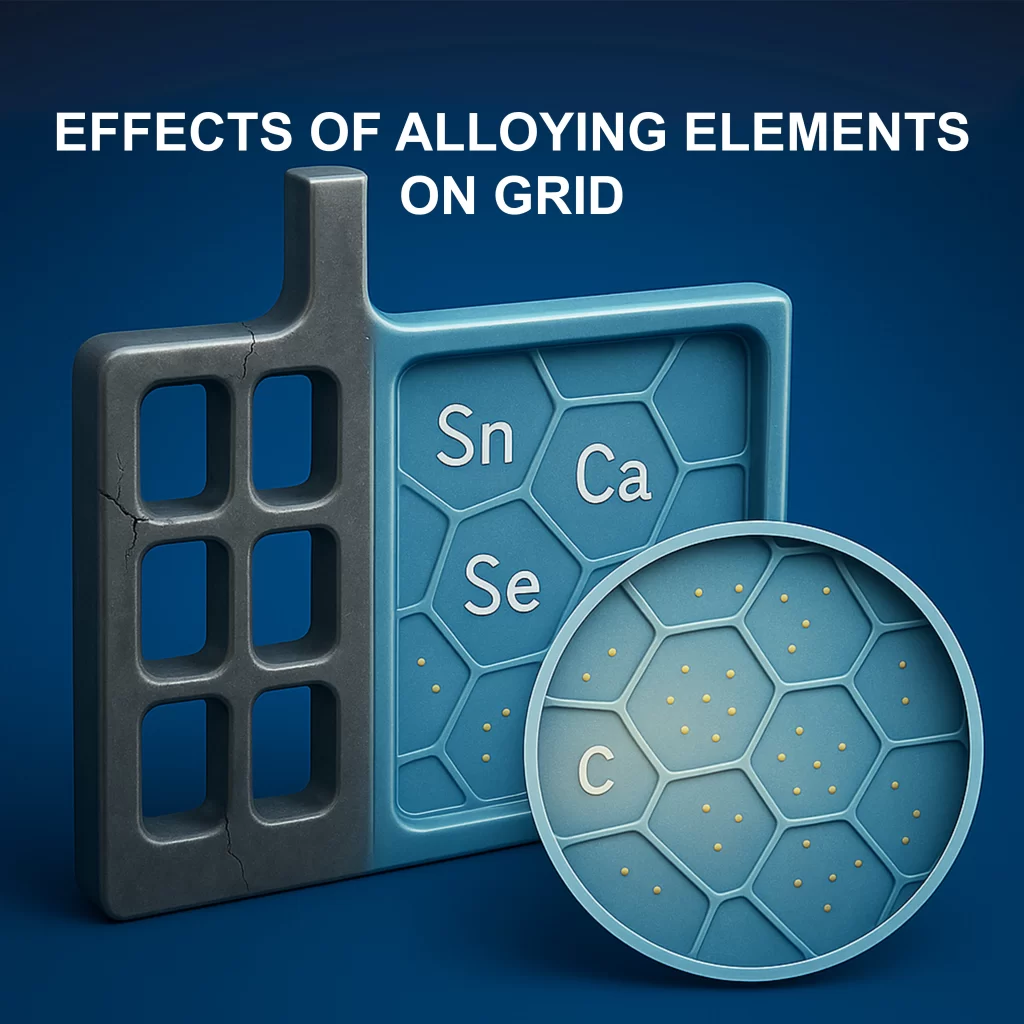
How Alloying Fights Corrosion
A Shift from Old Alloys
Early batteries used pure lead or toxic lead-antimony grids, which corroded quickly. Modern alloys with tin, calcium, and selenium are stronger, safer, and eco-friendly.
The Power of Alloying Elements
Alloying is like adding spices to a recipe, enhancing durability and performance:
• Tin (Sn): Shields grids from corrosion and boosts conductivity.
• Calcium (Ca): Strengthens grids and reduces maintenance.
• Selenium (Se): Refines structure for heat resistance.
Breaking Down the Elements
Tin: The Protective Shield
Tin (0.5–1.5%) forms a durable oxide layer, like a rust-proof coating, slowing corrosion. It also improves conductivity for reliable starts. Tests show tin extends grid life by up to 40% in tough conditions.
Calcium: The Strength Booster
Calcium (0.07–0.09%) replaces toxic antimony, hardening grids for lighter, longer-lasting batteries. It cuts water loss, reducing maintenance, and supports 99% recyclability for a greener footprint.
Selenium: The Heat Stabiliser
Trace amounts of selenium smooth the grid’s microstructure, like polishing a surface to prevent cracks. This boosts durability in hot climates or high-demand uses like solar storage.

How It Works
Corrosion occurs when acid attacks lead grids. Alloying elements create protective layers, like a sealed coat of paint, slowing degradation. Tin ensures conductivity, calcium limits growth, and selenium strengthens the structure.
Performance at a Glance
| Element | Benefits | Content | Without Element |
| Tin (Sn) | Corrosion resistance, conductivity | 0.5–1.5% | Faster corrosion, weak power |
| Calcium (Ca) | Strength, low maintenance, eco-friendly | 0.07–0.09% | Soft grids, high water loss |
| Selenium (Se) | Heat resistance, durability | Trace | Brittle grids, heat failure |
Real-World Proof
Lab and Field Data
• Tin-calcium grids last 40% longer in high-cycle tests.
• Selenium enhances performance in hot environments.
• Alloys are 99% recyclable, cutting lead waste by thousands of tonnes yearly.
Case Study: Fleet Savings
A fleet manager reported a 30% drop in battery replacement costs after switching to advanced grid batteries, thanks to longer service intervals and reduced downtime.

Eco-Friendly and Cost-Saving Benefits
• Safer Materials: Calcium and selenium replace toxic antimony.
• High Recyclability: 99% recyclable, reducing environmental impact.
• Low Maintenance: Less water top-up saves time and costs.
FAQs
Conclusion
Advanced grid alloying transforms traditional lead-acid batteries into high-performance, long-lasting, and eco-friendly energy systems. By incorporating tin, calcium, and selenium, these batteries resist corrosion, perform better in extreme conditions, and reduce overall ownership costs. Whether for automotive, industrial, or renewable energy use, modern alloyed grids redefine durability and sustainability in battery technology.
References:
- Lakshmi, C. S., Manders, J. E., & Rice, D. M. (1998).
Structure and properties of lead–calcium–tin alloys for battery grids.
Journal of Power Sources, 73(1), 23-29. ScienceDirect Link - Giess, H. (1995).
The influence of calcium, tin and grid thickness on corrosion-induced grid growth.
Journal of Power Sources, 53(1), 31–43. ScienceDirect Link - Rocca, E., Bourguignon, G., & Steinmetz, J. (2006).
Corrosion management of PbCaSn alloys in lead-acid batteries: Effect of composition, metallographic state and voltage conditions. Journal of Power Sources, 161(1), 666–675. ScienceDirect Link - Carreon, L. P. L., Guzman, M. A., Ejera, S. K. G., & Ong, A. C. (2015).
Effect of Tin Addition on the Corrosion Behavior of Lead-Calcium-Tin Grids in Lead-Acid Batteries. International Journal of Chemical Materials and Environmental Research, 3(2), 17-22. ResearchGate - Jullian, E., Albert, L., & Caillerie, J. (2003).
New lead alloys for high-performance lead–acid batteries.
Journal of Power Sources, 116(1), 185-192. ScienceDirect Link - Santos, A. G., et al. (2024).
The Effect of Ca and Bi Addition on the Mechanical Strength and Corrosion Resistance of Pb–Sn Alloys. Heliyon, 10, e38536. ScienceDirect Link