At SUZUKI Battery, innovation and reliability drive everything we do. One major advancement helping extend the lifespan and boost the performance of lead-acid car batteries is the smart use of battery additives.
These specially engineered compounds, added either to the active materials or the electrolyte, dramatically enhance battery function, ensuring your vehicle’s energy storage stays strong and efficient for the long haul.
Benefits of Battery Additives for Car Batteries
- Extended Battery Life: Helps prevent early wear and keeps your battery running longer.
- Improved Charge Acceptance: Enables faster and more complete recharging cycles.
- Reduced Sulfation: Stops large, damaging lead sulfate crystals from forming.
- Enhanced Electrical Conductivity: Improves electron flow for better battery performance.
- Stabilized Active Materials: Maintains strength and structure even during deep discharge cycles.
Technical Breakdown: How Battery Additives Work
1. Carbon Additives in Negative Plates
Enhancing Lead Deposition and Battery Conductivity
Carbon materials like graphite, activated carbon, and carbon nanotubes play a crucial role in battery performance. Acting as tiny nucleation sites, they help lead deposit more evenly during charging, reduce internal resistance, and prevent harmful lead sulfate buildup. The result? Faster charging, longer battery life, and stronger power output.
Figure 1 shows a SEM image of uniform lead nucleation on a carbon-enhanced substrate. The spherical lead deposits formed during charging demonstrate how carbon additives promote fine, even deposition, reducing localized stress and enhancing electrode electrochemical activity—key to improving battery cycle life, charge acceptance, and overall performance.
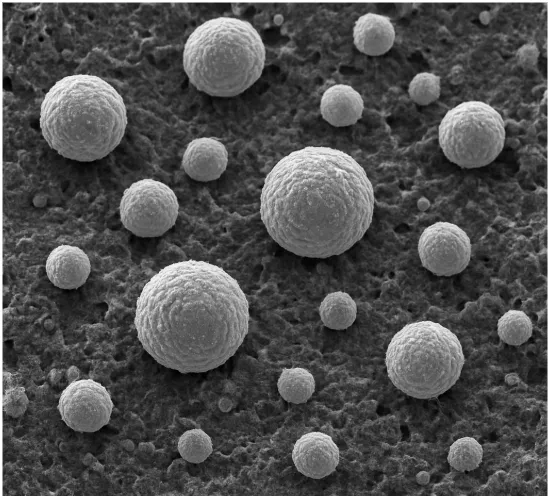
2. Tetrabasic Lead Sulfate (4BS) as a Positive Active Material
Building a Resilient Positive Electrode
Tetrabasic lead sulfate (4BS) crystals strengthen the positive active material by forming a tough lead dioxide structure that resists shedding and softening. This means better capacity retention and a battery that lasts significantly longer.
3. Zeolite and Graphite Additives
Creating Porous, Stable Structures
Adding zeolites and graphite creates a porous network that allows acid and ions to move more freely. This not only enhances mechanical stability under stress but also cuts down on material loss and acid layering, helping batteries perform better for longer.
4. Graphene Oxide with Lead Dioxide Nanoparticles
Dual Functionality for Enhanced Capacity and Stability
Blending lead dioxide nanoparticles into graphene oxide matrices does double duty: boosting electrical conductivity and offering excellent nucleation sites. This innovative method forms fine PbO₂ crystals, which improve both battery capacity and partial-state-of-charge performance.
5. Carbon Nanotubes for Advanced Electrode Reinforcement
Superior Conductivity and Structural Durability
Multi-wall carbon nanotubes (MWCNTs) bring exceptional electrical and mechanical properties to the table. By forming a highly conductive network inside the electrode, they curb sulfation and boost cycling stability, delivering higher power over longer periods.
6. Sustainable Carbon Materials: A New Frontier
Eco-Friendly Innovations like Carbonized Wool
New research into green alternatives like carbonized sheep’s wool shows big promise. These eco-friendly additives enhance adhesion and structural strength, offering a sustainable, cost-effective way to upgrade lead-acid battery performance.
Figure 2 highlights how engineered additives, such as advanced carbon materials and nanostructured compounds, enhance battery performance by improving conductivity, catalytic activity, and pore structure. Optimized properties like porosity and surface area lead to longer cycle life, better charge acceptance, and higher power density. Tailored additives consistently outperform conventional ones.
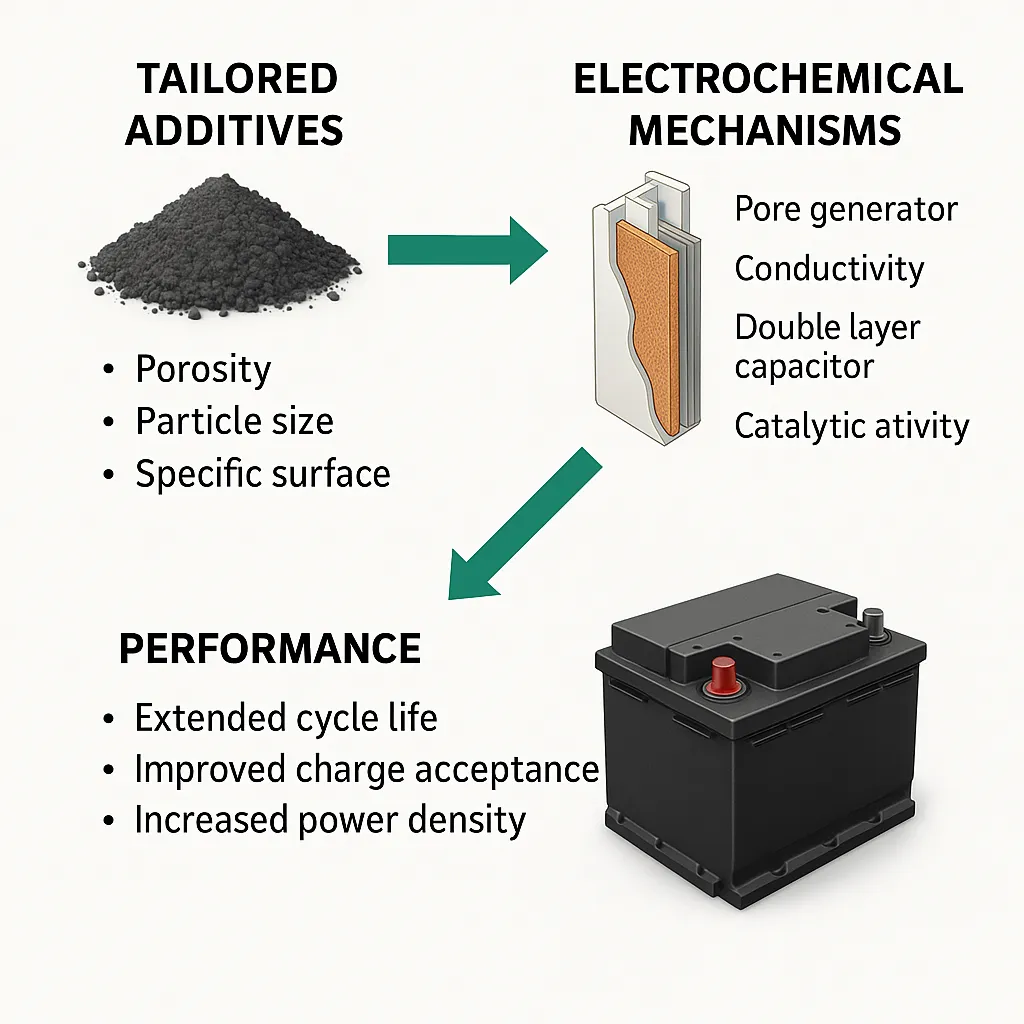
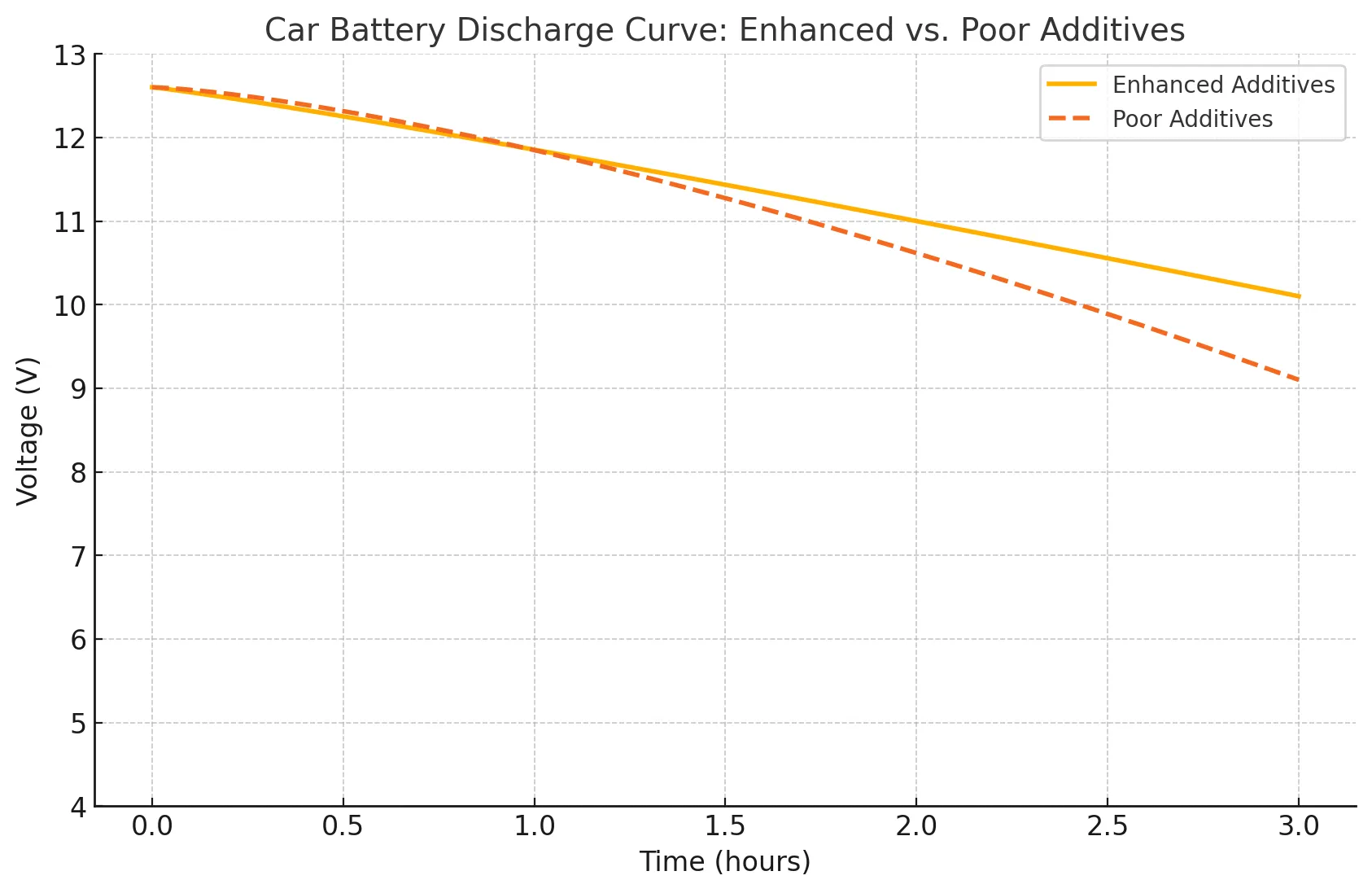
Figure 3 compares battery discharge performance between cells with optimized additives and those with poor-quality ones. Batteries with enhanced additives show slower voltage drop and better charge retention, while those with poor additives lose energy faster. This clearly shows how tailored additives improve endurance and stability over time.
FAQs: Battery Additives and Car Battery Performance
Conclusion: Elevate Your Drive with SUZUKI Battery Additives
Battery additives are a major leap forward in making car batteries last longer, perform better, and stay more reliable. At SUZUKI Battery, we use these advanced innovations to deliver batteries that meet the highest standards of performance and durability. Drive farther, charge faster, and enjoy greater peace of mind with SUZUKI Batteries.
References:
- Toussaint, G., et al. (2005). Effect of additives in compressed lead–acid batteries. Journal of Power Sources.
- Pavlov, D. (2017). Lead-Acid Batteries: Science and Technology. Elsevier.
- Valenciano, J., et al. (2009). Lead-acid batteries for micro- and mild-hybrid applications. Journal of Power Sources.
- Baraniak, M., et al. (2025). Novel carbon material in lead-acid battery technology. Monatshefte für Chemie.
- Cericola, D., & Spahr, M. (2016). Nucleation and electrolytic deposition of lead on model carbon electrodes. Journal of Power Sources.
- Ren, L., et al. (2023). Bifunctional additive: Lead dioxide nanoparticle-doped graphene oxide composites. Colloids and Surfaces A.
- Marom, R., et al. (2015). Enhanced performance of starter lighting ignition type lead-acid batteries with carbon nanotubes. Journal of Power Sources.

