Automotive and energy storage sectors worldwide demand efficient lead-acid batteries with advanced expanders. As vehicle electrification, renewable integration, and start-stop technologies continue to grow, traditional batteries face increased stress from frequent cycling and partial charging. This is where advanced additives—particularly expanders—play a vital role in modernising battery chemistry. This article explores how novel expanders work, what benefits they offer, and why they are essential to next-generation battery systems.
What Are Expanders?
Expanders are specialised additives mixed into the negative active material (NAM) of lead-acid batteries. Their primary function is to:
• Control the growth of lead sulfate (PbSO₄) crystals
• Maintain the porosity and conductivity of the negative plate
• Improve reversibility during charge and discharge cycles
Without expanders, lead sulfate crystals can grow excessively during discharge and become difficult to reconvert into lead during recharging, leading to capacity loss and sulfation—a major cause of battery failure.
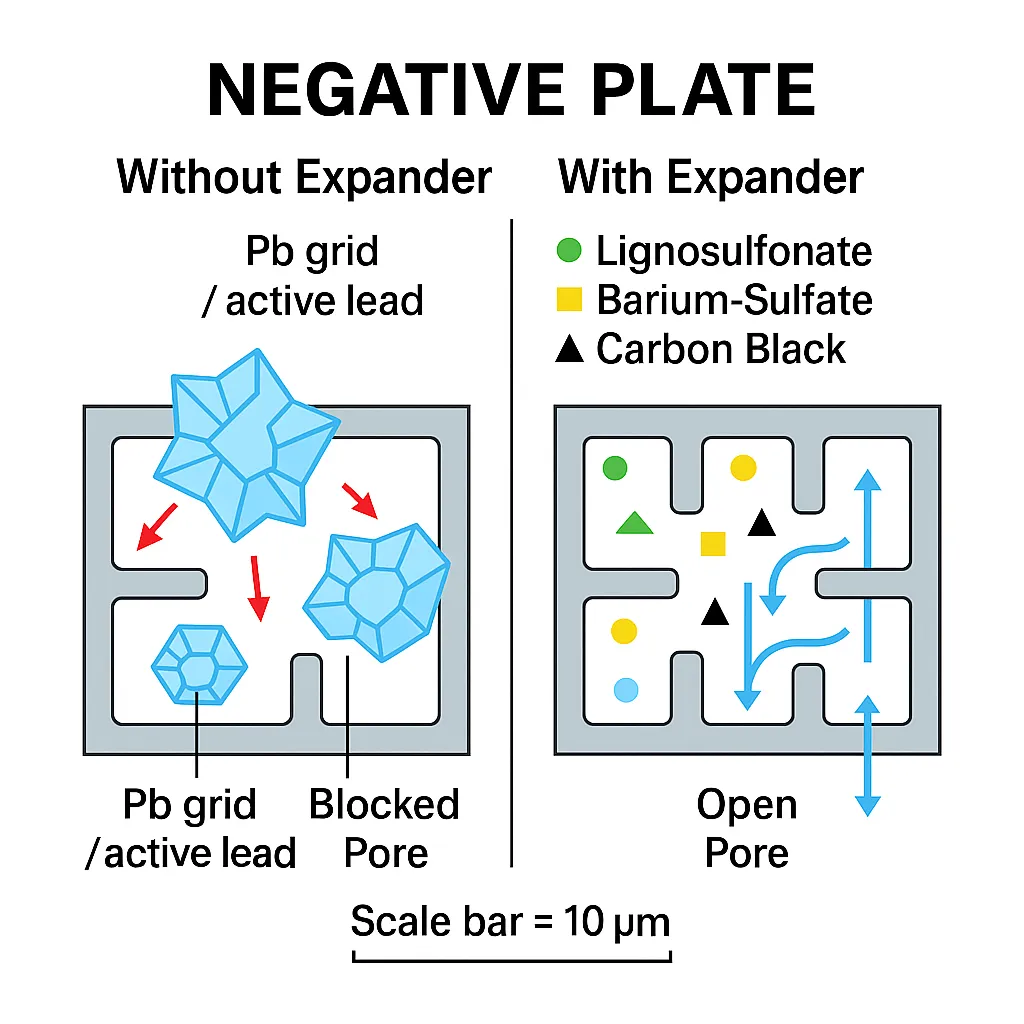
Typical Expander Components
Expanders typically include a blend of the following substances:
• Barium sulfate: Provides nucleation sites for uniform PbSO₄ formation
• Lignosulfonates and humic acids: Derived from wood or coal, they help maintain the sponge-like structure of lead
• Carbon black or graphite: Enhance conductivity and surface area
• Synthetic organic compounds: Improve charge efficiency and structural integrity
Modern formulations may also include silica, fibrous materials, or soluble organics to fine-tune pore structure and optimise electrochemical performance.
How Novel Expanders Improve Charge Acceptance
Recent advances have introduced carbon-based expanders, such as activated carbon, carbon nanotubes (CNTs), and MXene materials, to dramatically improve charge acceptance. These materials function not just as physical modifiers but as active electrochemical components.
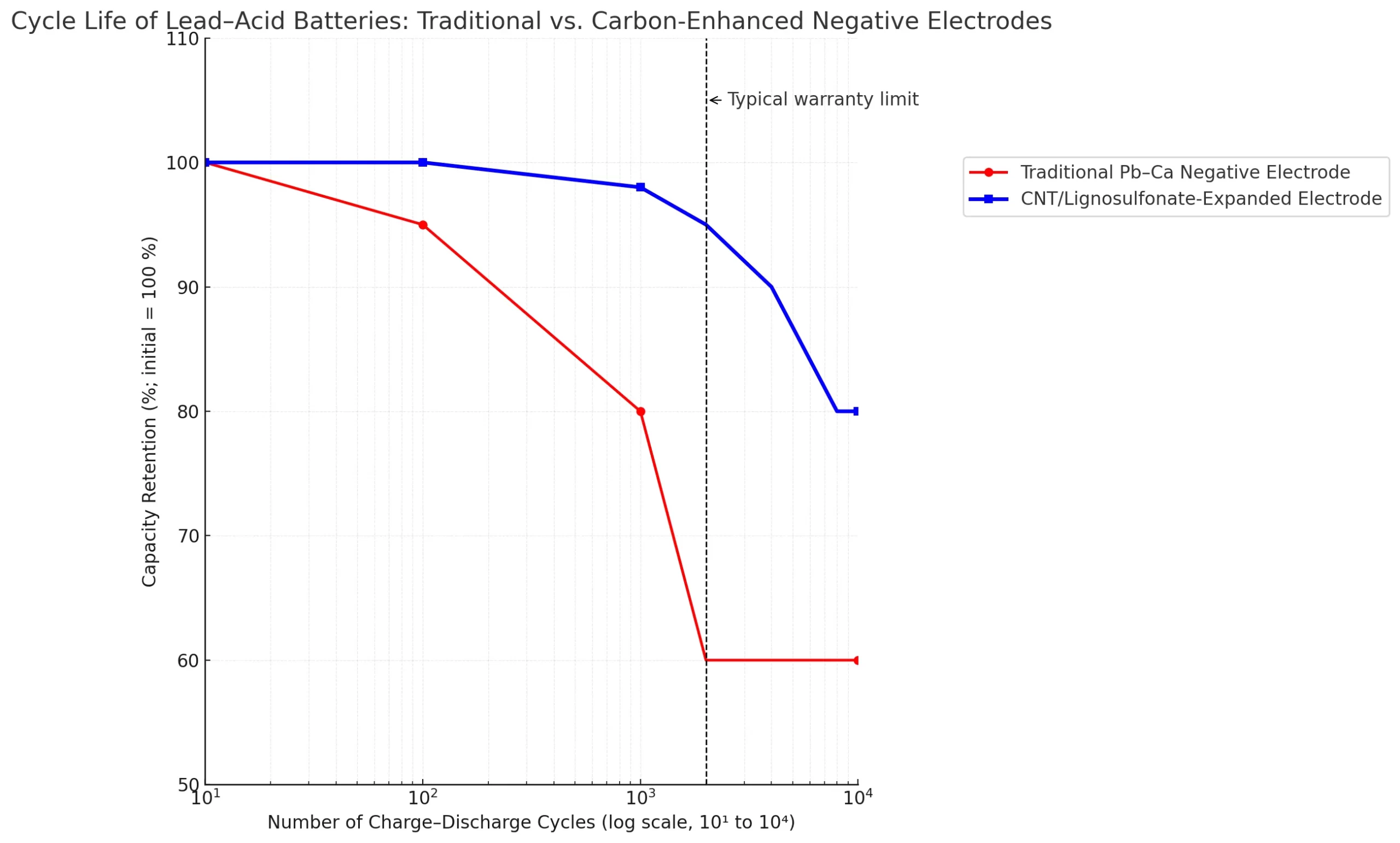
• Carbon-coated negative electrodes showed up to a threefold increase in cycle life and superior charge rates compared to conventional materials.
• Lead-carbon electrodes utilise activated carbon as an electron distributor, facilitating uniform lead deposition and inhibiting hydrogen evolution—both critical at high charge rates.
• Carbon nanotube expanders improved Cold Cranking Amps (CCA) and cycle life by over 60 % without affecting paste density or manufacturability.
• MXene-enhanced electrodes demonstrated superior dynamic charge acceptance (DCA) in rapid-screening tests, making them ideal for start-stop applications.
These innovations represent a shift from passive expander roles to active control of charge kinetics, ion movement, and electrode surface chemistry.
Technical Mechanisms Behind Performance Boost
The performance gains offered by novel expanders are supported by multiple mechanisms that enhance the battery’s electrochemical and structural properties:
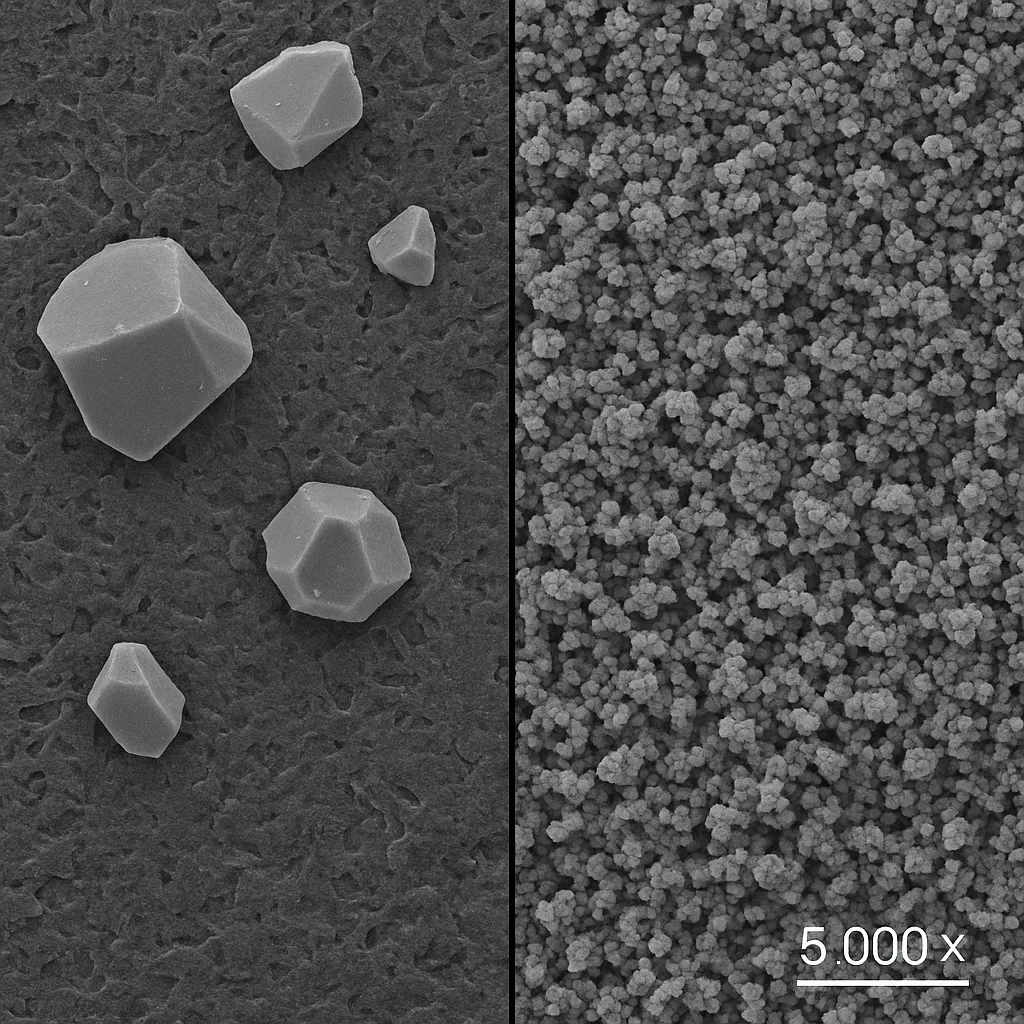
- Increased Surface Area
Additives like activated carbon and graphite significantly increase the electrode’s surface area, providing more active sites for lead deposition and ion exchange.
• Leads to better dynamic charge acceptance and reduces polarization losses. - Improved Lead Affinity and Nucleation
Carbon materials with good lead affinity improve nucleation behaviour, leading to finer PbSO₄ crystals and better reversibility during cycling.
• Expanded graphite offers more nucleation sites than synthetic graphite. - Hydrogen Suppression
Some novel expanders inhibit the hydrogen-evolution reaction (HER), a parasitic process that wastes energy and causes water loss. By reducing HER, batteries maintain better electrolyte levels and increase efficiency. - Enhanced Conductivity
Carbon-based expanders improve electron flow within the electrode matrix, reducing internal resistance and enhancing performance during high-rate charging. - Self-Healing Interfaces
New research into reversible ion adsorption and surface restructuring shows potential for self-healing properties that stabilise electrodes under cyclic stress. While still emerging, these mechanisms point to longer-lasting batteries with more robust electrodes.
Benefits for Battery Systems
Incorporating novel expanders in negative electrodes offers a wide range of benefits for battery systems used in automotive and industrial environments:
Performance
• Faster charging and enhanced dynamic charge acceptance (DCA)
• High-rate partial state of charge (HRPSoC) resilience for stop-start vehicles
Longevity
• Extended cycle life, especially in partial charge cycling
• Minimised sulfation through controlled crystal growth
• Resistance to capacity degradation from repetitive cycling and temperature fluctuations
Reliability
• Better low-temperature performance, crucial for cold climates
• Improved cold-cranking performance, thanks to more efficient electrode conductivity
• Consistent performance under load, vital for backup and standby systems
Efficiency
• Lower internal resistance and energy loss
• Reduced water loss, lowering maintenance requirements
• Enhanced coulombic efficiency, reducing power waste
Frequently Asked Questions
Conclusion
As the demand for efficient, long-lasting energy storage grows, novel expander technologies for lead-acid batteries offer a cost-effective, proven solution. By enhancing the charge acceptance of negative electrodes, these materials significantly extend cycle life and improve performance across applications—from stop-start vehicles to industrial backup systems and renewable energy storage.
Continued innovation in expander chemistry—particularly at the molecular and nano-scale—will be key to meeting future energy demands. SUZUKI Battery is at the forefront of this progress, working with materials scientists and OEMs to engineer cutting-edge solutions that push performance boundaries.
References
- High charge acceptance through interface reaction on carbon-coated negative electrode for advanced lead-carbon battery system, Sadhasivam, T., Park, M., Shim, J., et al., Electrochimica Acta, 2019. DOI: 10.1016/j.electacta.2018.10.149
- Zhang, W., Lin, H., Lu, H., et al. “On the Electrochemical Origin of the Enhanced Charge Acceptance of the Lead–Carbon Electrode.” J. Mater. Chem. A, 2015. DOI: 10.1039/C4TA05891G
- Sugumaran, N., Everill, P., Swogger, S. W., Dubey, D. “Lead-Acid Battery Performance and Cycle Life Increased Through Addition of Discrete Carbon Nanotubes to Both Electrodes.” Journal of Power Sources, 2015. DOI: 10.1016/j.jpowsour.2014.12.117
- Settelein, J., Lorrmann, H., Sextl, G. “Evaluating the Lead Affinity of Graphite Additives in Lead-Acid Batteries by Electrochemical Deposition.” Electrochimica Acta, 2017. DOI: 10.1016/j.electacta.2017.03.034
- Settelein, J., Oehm, J., Bozkaya, B., et al. “The External Surface Area of Carbon Additives as Key to Enhance the Dynamic Charge Acceptance of Lead-Carbon Electrodes.” Journal of Energy Storage, 2018. DOI: 10.1016/j.est.2017.11.016