A car battery is an essential component that delivers the electrical power required to start the engine and operate crucial systems such as lights, infotainment, and safety features. Lead–acid batteries, which have been the dominant choice in most motor vehicles for over a century, remain a reliable and cost-effective solution despite the emergence of newer technologies like lithium-ion.
Understanding their structure and underlying chemistry helps drivers, vehicle technicians, and automotive enthusiasts maintain optimal performance and prevent common issues such as starting failures or electrical malfunctions.
This guide explains how lead–acid car batteries work, examines their key components, and highlights the advantages they offer in automotive applications.
What Is a Lead–Acid Car Battery?
A lead–acid car battery is a rechargeable electrochemical device that stores and supplies electricity to start a vehicle’s engine, stabilise voltage, and power accessories such as GPS systems, USB ports, and electric windows. As batteries age or deteriorate, motorists may notice slow engine cranking, dim headlights, or dashboard error messages. These symptoms highlight the importance of understanding the battery’s operation, maintenance requirements, and replacement intervals.
How Lead–Acid Batteries Work: The Chemistry
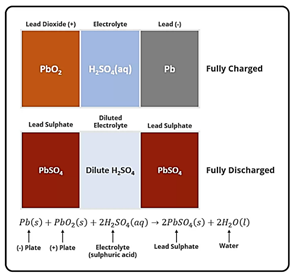
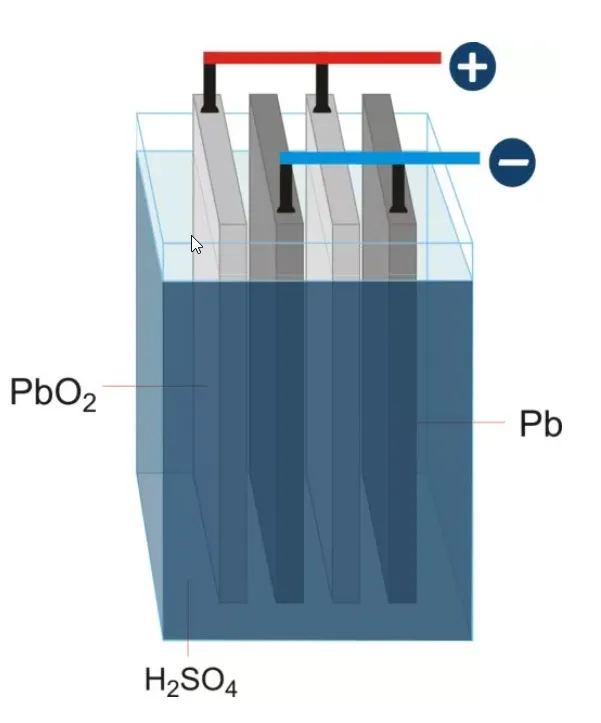
Lead–acid batteries generate electricity through electrochemical reactions between lead-based plates and a sulphuric acid (H₂SO₄) electrolyte. Each battery contains several cells, with each cell producing approximately 2.1 V. Combined, a fully charged six-cell battery delivers around 12.6 V.
Discharge Process
When a load—such as the starter motor—is connected:
- Positive plates made of lead dioxide (PbO₂) and negative plates made of sponge lead (Pb) react with the electrolyte (sulphuric acid, H₂SO₄).
- Both plates are converted to lead sulphate (PbSO₄), producing water (H₂O) and releasing electrons.
- These electrons flow through the vehicle’s electrical system, providing the energy needed to operate components.
Key Discharge Reaction:
PbO₂ + Pb + 2 H₂SO₄ → 2 PbSO₄ + 2 H₂O + electrical energy
Recharge Process
When the engine runs, the alternator reverses the reaction, restoring the plates to their original state and replenishing the electrolyte:
- Lead sulphate is converted back into lead dioxide (PbO₂) on the positive plates and sponge lead (Pb) on the negative plates.
- Sulphuric acid concentration in the electrolyte is restored, ensuring maximum capacity.
Key Recharge Reaction:
2 PbSO₄ + 2 H₂O → PbO₂ + Pb + 2 H₂SO₄ (with alternator input)
Failure to recharge the battery regularly can lead to sulphation—the formation of hardened lead sulphate crystals—which reduces capacity, shortens service life, and compromises reliability.
Lead–Acid Battery Structure
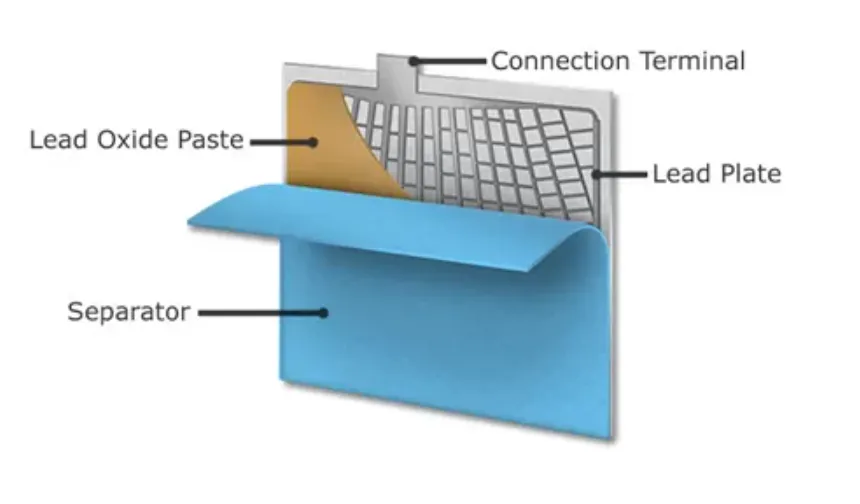
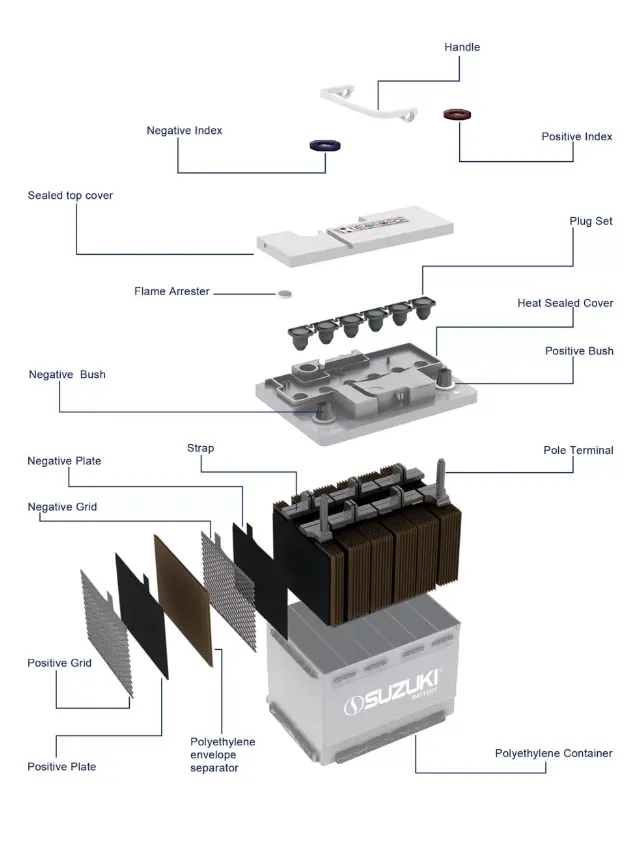
The design of a lead–acid car battery is engineered to deliver durability, efficiency, and operational safety, ensuring reliable performance in diverse driving conditions. Its key components include:
- Six-Cell Configuration: Six electrochemical cells, each producing approximately 2.1 V, combine to provide a total of around 12.6 V when fully charged.
- Plates and Active Material: Positive plates consist of lead dioxide (PbO₂), while negative plates use sponge lead (Pb). Both are coated with an active material to maximise the electrochemical reaction surface area.
- Separators: Thin, porous polyethylene sheets placed between plates prevent internal short circuits while permitting the free flow of ions.
- Electrolyte: A precisely formulated mixture of high-purity sulphuric acid (H₂SO₄) and deionised water drives the battery’s electrochemical processes.
- Battery Case: A corrosion-resistant polypropylene or polyethylene casing protects the internal components, withstands vibration, and endures extreme temperatures.
- Straps and Terminals: Heavy-duty lead straps connect the tops of the plates for current collection, while robust terminals ensure secure integration with the vehicle’s electrical system.
- Valve/Cap System: Flooded lead–acid batteries feature removable caps for maintenance, whereas valve-regulated lead–acid (VRLA) designs use sealed vents to manage internal gas pressure and enhance safety.

Lead–Acid vs. Lithium–Ion Batteries
| Feature | Lead–Acid | Lithium–Ion |
| Cost | Low – affordable to produce and purchase | High – higher initial investment |
| Energy Density | Moderate – larger size for same capacity | High – compact and lightweight |
| Cycle Life | 300–500 charge/discharge cycles | 1,000+ cycles |
| Temperature Tolerance | Excellent – tolerates hot and cold extremes | Sensitive to temperature extremes |
| Maintenance | Sealed (maintenance-free) or flooded (top-ups) | Fully maintenance-free |
| Charging Time | 4–8 h | 1–3 h |
| Safety | Stable, low risk | Risk of thermal runaway if damaged |
Key Benefits of Lead–Acid Car Batteries
- Affordable: Lower manufacturing and purchase costs compared with lithium-ion batteries.
- Proven reliability: Over a century of automotive use with consistent performance.
- High surge currents: Ideal for starter motors requiring instant power.
- Recyclable: Over 95 % of lead–acid batteries are recycled globally.
- Widely available: Easy to source and replace worldwide.
- Versatile designs: Available in flooded, AGM, and gel-cell variants to meet diverse needs.
Frequently Asked Questions (FAQs)
Conclusion
Lead–acid car batteries remain the backbone of automotive power, offering a cost-effective, reliable, and recyclable solution for starting engines and powering accessories. Understanding their chemistry and structure—plates, electrolyte, and robust casing—helps you maintain your battery and avoid issues such as sulphation or failure to start. Whether for a daily commuter or a heavy-duty vehicle, lead–acid batteries deliver proven performance tailored to your needs.
References
- Yamaguchi, Y. (2014). Lead Acid Batteries. In: Garche, J. (eds) Encyclopedia of Applied Electrochemistry. Springer, New York. Springer Nature Link
- Petrovic, S. (2020). Lead–Acid Batteries. In: Garche, J. (eds) Encyclopedia of Electrochemical Power Sources. Springer, Cham. Springer Nature Link
- Bullock, K. (1994). Lead/Acid Batteries. Journal of Power Sources, 53(1), 39–48. Science Direct
- Li, G., Cao, X., & Liu, H. (2022). Application and Development of Lead–Carbon Battery in Electric Energy Storage System. EasyChair Preprint
- Frost, P. (1999). Developments in Lead–Acid Batteries: A Lead Producer’s Perspective. Journal of Power Sources, 78(1–2), 9–12. Science Direct
- Pavlov, D. (2017). Lead–Acid Batteries: Science and Technology. Elsevier. ISBN 978-0444595522.
- Mahmoud, T. H. & Xu, L. (2011). A Combined Li-ion & Lead-Acid Battery System for Start-Stop Application: Potential & Realization. Chalmers University of Technology.

