The lead-acid battery has a storied history stretching over a century, embodying the culmination of scientific discovery and technological innovation. This article delves into the rich history, intricate functionality, and diverse types of lead-acid batteries, focusing on their critical role in modern automotive applications and beyond.
- The Historical Development of Lead-Acid Batteries: From Early Discoveries to Groundbreaking Innovations
- How Lead-Acid Batteries Work: The Chemistry Behind Powering Your Vehicle
- The Role of the Electrolyte: Sulfuric Acid as the Lifeblood of Lead-Acid Batteries
- Types of Lead-Acid Batteries: Customizing Performance for Specific Needs
- Beyond Starting: Lead-Acid Batteries for Specialized Applications
- The Science of Lead-Acid Battery Voltage: Understanding the Nernst Equation
- Advanced Lead-Acid Battery Technologies: VRLA and the Role of Leady Oxide
- The Future of Lead-Acid Batteries: Innovations and Emerging Trends
- Conclusion: The Enduring Legacy of Lead-Acid Batteries
The Historical Development of Lead-Acid Batteries: From Early Discoveries to Groundbreaking Innovations
The lead-acid battery, one of the earliest forms of rechargeable energy storage, has its roots in the early 19th century. As electricity began to revolutionize society, pioneering scientists like Nicholas Gautherot and Johann Ritter conducted experiments that laid the groundwork for future advancements. Gautherot’s discovery of a brief reverse current and Ritter’s identification of residual voltage and material polarization were crucial steps leading to the eventual creation of the lead-acid battery.

In 1859, Gaston Planté made a monumental leap in energy storage technology by inventing the first rechargeable lead-acid battery. His design, featuring lead plates immersed in sulfuric acid, offered a reliable, reusable power source that would later become indispensable in automotive technology and numerous other applications.
How Lead-Acid Batteries Work: The Chemistry Behind Powering Your Vehicle
A lead-acid battery operates through electrochemical reactions involving lead dioxide (PbO₂) and pure lead (Pb) plates submerged in sulfuric acid. This interaction during the charge and discharge cycles allows the battery to store and deliver electrical energy efficiently.
Discharge Process (Power Supply):
- Anode (Lead Plate): The pure lead plate reacts with sulfuric acid, forming lead sulfate (PbSO₄) and releasing electrons.
- Cathode (Lead Dioxide Plate): The lead dioxide plate consumes these electrons and sulfuric acid to produce lead sulfate, water, and energy.
During discharge, the overall chemical reaction transforms the lead and lead dioxide into lead sulfate and water, releasing electrical energy to power the vehicle.
Recharge Process (Restoring Power)
- Anode (Lead Plate): During recharging, lead sulfate and hydrogen ions convert back into pure lead and sulfuric acid.
- Cathode (Lead Dioxide Plate): Lead sulfate, water, and electrons convert back into lead dioxide, sulfuric acid, and hydrogen ions.
This reversible reaction makes lead-acid batteries rechargeable, though repeated cycles gradually reduce the battery’s capacity over time.
The Role of the Electrolyte: Sulfuric Acid as the Lifeblood of Lead-Acid Batteries
In lead-acid batteries, sulfuric acid acts as the electrolyte, facilitating the necessary chemical reactions between the lead and lead dioxide plates. Unlike other battery types that require a salt bridge to maintain ion flow, lead-acid batteries use direct contact between the electrolyte and the plates. This design simplifies the battery structure while ensuring efficient ionic movement and energy conversion.
Types of Lead-Acid Batteries: Customizing Performance for Specific Needs
Lead-acid batteries have been adapted into various forms, each tailored to meet specific demands. Below is an exploration of the most common types of lead-acid batteries and their applications:
- Starter Batteries (SLI Batteries):
- Purpose: These batteries provide a burst of high current to start engines.
- Characteristics: Featuring many thin plates, they deliver short bursts of power but are not suited for deep cycling.
- Applications: Primarily used in vehicles such as cars, motorcycles, and trucks.
- Micro-Cycle Batteries:
- Purpose: Designed for vehicles with start-stop technology, which reduces idle time and improves fuel efficiency.
- Characteristics: These batteries can endure frequent and shallow charge-discharge cycles, tailored for start-stop functionality.
- Applications: Common in modern vehicles with start-stop systems.
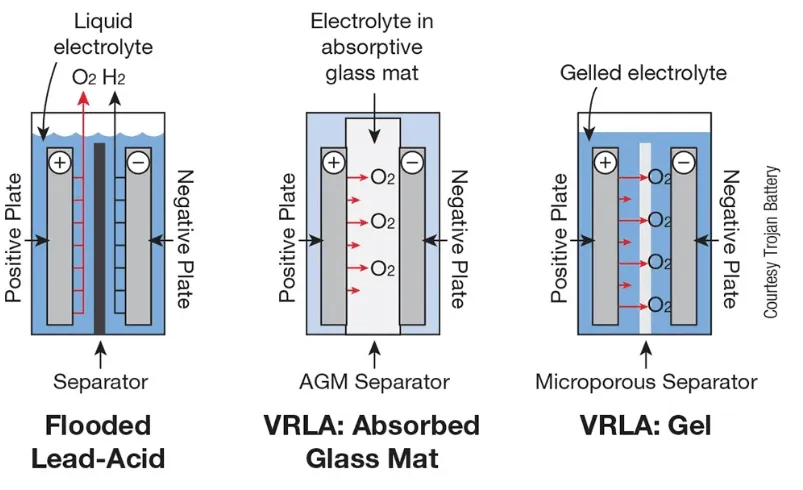
- Flooded Lead-Acid Batteries:
- Characteristics: Traditional batteries with a liquid electrolyte, requiring regular maintenance such as water top-ups to compensate for water loss during electrolysis.
- Applications: Frequently used to start engines in various vehicles, given their ability to deliver the high current necessary for cranking.
- Valve-Regulated Lead-Acid (VRLA) Batteries:
- Absorbed Glass Mat (AGM) Batteries:
- Characteristics: These batteries contain an electrolyte absorbed into fiberglass mats, making them spill-proof and reducing maintenance.
- Applications: Common in vehicles requiring higher vibration resistance, such as off-road or luxury vehicles.
- Gel Batteries:
- Characteristics: Although Gel batteries are a type of VRLA battery, they are generally not used for starting applications due to their deep cycle design.
- Applications: Frequently used in marine settings, wheelchairs, and renewable energy installations.
- Absorbed Glass Mat (AGM) Batteries:
Beyond Starting: Lead-Acid Batteries for Specialized Applications
While starter batteries are the most well-known type, several other types of lead-acid batteries are designed for more specific uses:
- Deep Cycle Batteries:
- Purpose: Built to deliver a steady current over a long period, these batteries have thicker plates that withstand repeated cycling.
- Applications: Ideal for electric vehicles, golf carts, marine applications, and off-grid renewable energy systems.
- Stationary Batteries:
- Purpose: Designed for stationary settings, these batteries provide reliable power or backup power for extended periods.
- Applications: Common in telecommunications, power generation, and emergency backup systems such as UPS (Uninterruptible Power Supplies).
- Gel Cell Batteries:
- Purpose: These VRLA batteries use a gelified electrolyte, making them highly resistant to leaks and evaporation.
- Applications: Often found in marine environments, wheelchairs, and renewable energy installations.
The Science of Lead-Acid Battery Voltage: Understanding the Nernst Equation
The voltage produced by a lead-acid battery is a key factor in its operation, determined by the chemical reactions within the battery. While the standard electrode potential for these reactions is around 2.1 volts, this value is somewhat idealized, assuming standard conditions like concentration and pressure.
To calculate the actual cell potential (E) under real conditions, the Nernst equation is used, accounting for factors such as the concentration of sulfuric acid in the electrolyte. Here’s a simplified explanation:
- Standard Electrode Potential (E°): For a lead-acid battery, this is approximately 2.04 V.
- Nernst Equation: The equation used is:
- E = E° – (0.0592/n) log ( (H₂O)² / [H₂SO₄]² )
- where n = 2 (the number of electrons transferred).
- Example Calculation:
- With a sulfuric acid concentration [H₂SO₄] = 4.5 M and the activity of water [H₂O] approximated as 1, the cell potential E would be approximately 2.0787 V.
Understanding the Nernst equation and its implications helps in optimizing the battery’s performance, ensuring it meets the demands of its specific application.
Advanced Lead-Acid Battery Technologies: VRLA and the Role of Leady Oxide
Valve-Regulated Lead-Acid (VRLA) batteries represent an advanced type of lead-acid battery, known for their sealed design and reduced maintenance requirements. A critical factor influencing the performance of these batteries is the leady oxide used in manufacturing the plates. The particle size distribution, specific surface area, and phase composition of the leady oxide directly affect the structure and morphology of the active materials within the plates.
For instance, finer leady oxide particles contribute to a higher initial battery capacity but may result in a shorter cycle life. On the other hand, coarser particles might lead to a lower initial capacity but a more gradual decline in performance, thereby extending the battery’s cycle life. The meticulous control of these properties during the manufacturing process is essential to optimize battery performance and ensure long-term reliability.
The Future of Lead-Acid Batteries: Innovations and Emerging Trends
As the world continues to seek more efficient and sustainable energy solutions, the role of lead-acid batteries remains significant. Despite the rise of newer technologies like lithium-ion batteries, lead-acid batteries offer unique advantages, particularly in terms of cost, recyclability, and reliability.
Future innovations in lead-acid battery technology are likely to focus on improving energy density, reducing weight, and enhancing cycle life. Additionally, advancements in materials science could lead to the development of hybrid systems that combine the benefits of lead-acid batteries with those of other energy storage technologies.
Conclusion: The Enduring Legacy of Lead-Acid Batteries
The evolution of the lead-acid battery, from its early beginnings to its current role in modern technology, is a remarkable story of human ingenuity and collaboration. These batteries have powered vehicles, supported critical infrastructure, and enabled countless technological advancements. As we look to the future, the legacy of the lead-acid battery will continue to influence the development of new energy storage solutions, ensuring its place as a cornerstone of modern industry.
Modern lead-acid batteries, with their proven reliability and adaptability, remain a vital component in the automotive industry and beyond. Whether powering vehicles, supporting renewable energy systems, or providing backup power in critical applications, the lead-acid battery\’s role is as essential today as it was over a century ago.
