One of the vital components of a vehicle is its battery. The battery is essential not only for starting the engine but also for powering the vehicle’s electrical equipment.
With advancements in technology, there are various types of car batteries with different features and applications.
The evolution of car batteries has focused on four key areas: improved performance and safety, increased range, faster charging, and enhanced efficiency in generating electrical energy.
The History and Roots of the Car Battery
Historical evidence suggests that the first man-made battery was used by the Parthians, who inhabited the regions of present-day Iran and Iraq around 200 BC.
In 1936, during excavations for railway lines near Baghdad, workers discovered pottery vessels approximately 12 centimeters in size.
After thorough investigation and experimentation, it was determined that these vessels had a function similar to modern batteries.

Inside these vessels, an iron rod served as the positive terminal, placed within a copper cylinder serving as the negative terminal, and a vinegar-like solution was used as an electrolyte. Reproducing these samples in a laboratory demonstrated that this setup could produce a voltage between 1.1 up to 2 volts. It seems the invention of the battery back to about 2000 years ago.
The First Mass-Produced Battery
The evolution of the car battery began with the invention of the first battery by Alessandro Volta in 1799. He discovered that certain liquids could produce a continuous electric current when used as a conductor.
He then realized that different metals could acquire and release electrons at varying speeds (voltage potential).
In the next stage, Volta succeeded in creating the first battery by placing two electrodes made of different metals in an electrolyte solution within a container (cell).
Ultimately, Volta discovered that the voltage could be increased by adding more cells containing the electrolyte and two different electrodes.
The work of Volta and other scientists such as Anthony Carlisle and William Nicholson led to the design of car batteries.
The first mass-produced battery was designed and built by a chemist named William Cruickshank in 1802.
The structure of the battery evolved in the following years, but these batteries were not rechargeable and were disposable.

The First Lead-Acid Battery
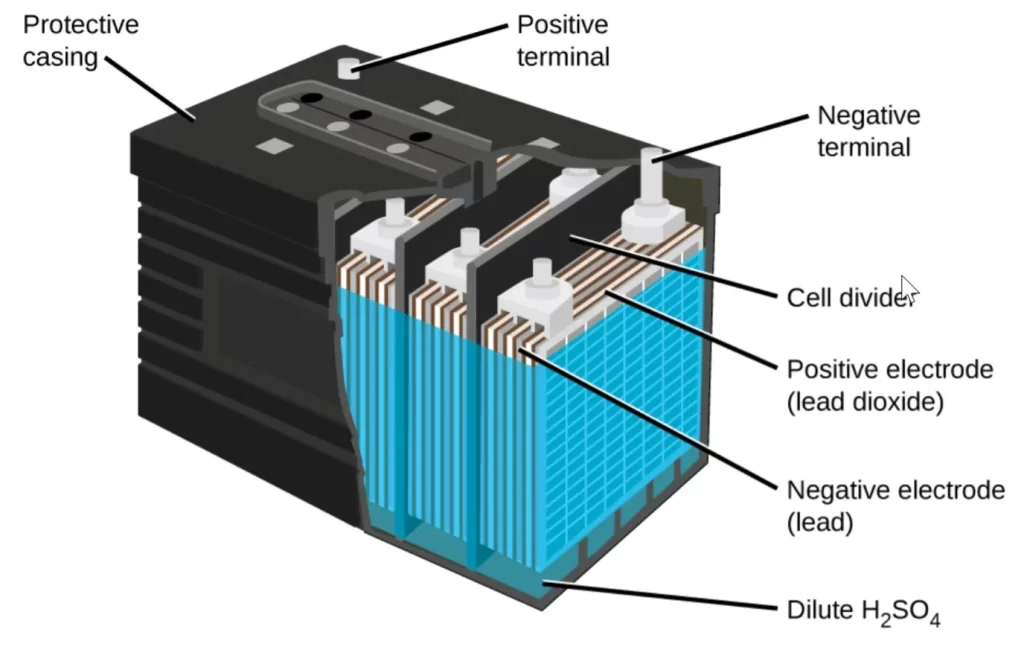
By 1859, French physicist Gaston Planté solved the issue of disposable batteries by introducing the first lead-acid battery. Planté used lead and lead dioxide as the positive and negative electrodes in his battery.
This was the first battery in history that utilized a single electrolyte for both electrodes. The most significant advantage of lead-acid batteries was their ability to be recharged by reversing the chemical reaction.
Despite all the limitations and shortcomings, such as the short charging duration, this design represented a major step in the evolution of automotive batteries. In 1881, a French chemist named Camille Alphonse Faure improved Plant’s invention by creating a better structure for the battery.
Instead of Plant’s spiral design, Faure created a lead grid where lead dioxide plates were mechanically pressed onto it. This innovative design allowed multiple plates to be combined for higher potential and greater power, and also made mass production easier and more justifiable.
The Role of the Electric Starter in the Development of the Automotive Industry and Transportation
Significant changes were made to the early designs of Plant’s battery from 1859 until the early 20th century, but the first car equipped with an electric starter was produced and brought to market in 1912.
Prior to this, vehicles predominantly used internal combustion engines with manual starters, which posed many challenges and difficulties for drivers.
The electric starter system allowed drivers to start the engine with the press of a button, which significantly improved the convenience and efficiency of driving and led to greater popularity for lead-acid batteries.
Evolution of Lithium Batteries
Over time, and with technological advancements, batteries with better performance became necessary. In the 1990s, lithium battery technology was introduced to the market, offering many advantages over lead-acid batteries.
Lithium batteries are lighter, have a higher energy density, and have a longer life cycle in terms of charging and discharging.
This technology was rapidly adopted in electric and hybrid vehicles, enabling the development of cars with better performance and more affordable prices.
Today, lithium batteries, particularly lithium-ion types, are used in many new and electric vehicles and are considered key components in the design and production of modern cars.
A Brief Overview of Common Types of Automotive Batteries
Lead-Acid Batteries
This type of battery is one of the most common types of automotive batteries. Lead-acid batteries are primarily divided into two categories:
Conventional Batteries
These batteries do not require continuous maintenance and are composed of acid and lead. They are widely used in both old and new vehicles.
Absorbent Glass Mat (AGM) Batteries
These batteries are typically used for vehicles with more electrical tools and sensitive equipment. AGM batteries utilize the addition of glass fibers to the electrolyte to improve performance and reduce acid leakage.
Lithium-Ion Batteries
Lithium-ion batteries are used in electric and hybrid vehicles due to their lightweight and longer lifespan.
These batteries have a greater energy storage capacity compared to lead-acid batteries and have shorter charging times.
Nickel-Metal Hydride (NiMH) Batteries
Nickel-Metal Hydride (NiMH) batteries are commonly used in hybrid vehicles. They can store a significant amount of electrical energy and have a longer lifespan than lead-acid batteries.
Gel or Gel-Acid Batteries
These batteries, also known as gel cell batteries, are a type of sealed lead-acid battery that utilize a silica-based gel electrolyte. This design allows gel batteries to be maintenance-free, leak-proof, and capable of withstanding deep discharges without damage.
They generally have a longer lifespan compared to traditional lead-acid batteries with liquid electrolytes and are ideally suited for automotive, marine, and solar energy storage applications.
Due to their reliable performance and resistance to shocks and vibrations, they are highly popular among users.
Although gel batteries are typically more expensive than conventional lead-acid batteries and require specialized chargers, their advantages—such as low discharge rates, greater safety, and resistance to high temperatures—make them a preferred choice for those seeking durable and efficient energy storage solutions for specific conditions.
Lithium Iron Phosphate (LiFePO4) Batteries
These types of batteries are used in some electric vehicles and electric buses due to their high temperature tolerance and long lifespan. One of the other positive aspects of these batteries is their high safety.
Considerations for Choosing the Right Battery for Your Vehicle
Assess Vehicle Needs
Before purchasing a battery, it is essential to consider the specific needs of your vehicle. For example, if your vehicle is a newer model with advanced electrical equipment, you should select a battery with a higher capacity compared to other cars.
Check Battery Capacity and Amperage
Battery capacity refers to the amount of energy stored in it and is usually expressed in amp-hours (Ah).
You must ensure that the capacity of the chosen battery meets the requirements of your vehicle and its electrical equipment. For instance, vehicles with powerful sound systems or additional lighting systems may require higher-capacity batteries.
Check Battery Size
Batteries come in various sizes. Before purchasing, identify the size of your current vehicle battery and ensure that the dimensions of the new battery are compatible with the available space in your vehicle’s battery compartment.
Weather Conditions
The climate in your area can also affect battery performance. AGM batteries generally perform better in various temperatures during winter and summer. In regions with high temperatures, conventional batteries typically have a shorter lifespan compared to other areas.
The main reasons for the reduced lifespan of batteries in hot climates include prolonged use of air conditioning and increased evaporation of the car battery’s electrolyte.
Safety Features
Some batteries come with specific safety features that can prevent damage to the vehicle in case of danger.
For example, AGM batteries reduce the risk of acid leakage and are generally safer.

Financial Considerations
You should set a budget for purchasing a battery. Although lithium-ion batteries are more expensive, they have greater durability and a longer lifespan, which may make them more cost-effective in the long run. Lead-acid batteries are typically more economical but have a shorter lifespan.
Warranty and Guarantees
Car battery warranties are mainly replacement warranties for a specified period due to manufacturing defects. The warranty duration is usually determined by the manufacturer based on the quality of the battery, its price, and, of course, the climate conditions.
Generally, batteries are warranted for an average of 12 to 18 months in moderate regions, while in cold areas, they can be warranted for up to three years (or more). In tropical regions, however, car battery warranties are typically limited to a maximum of 12 months.
Conclusion
Choosing the right battery significantly contributes to better performance and the longevity of the vehicle. It is always best to purchase batteries from reputable brands with suitable warranties. Being informed about the opinions of others, especially automotive electrical specialists, can also greatly assist in selecting the appropriate battery.